Which of the Following Would Have the Highest Vapor Pressure
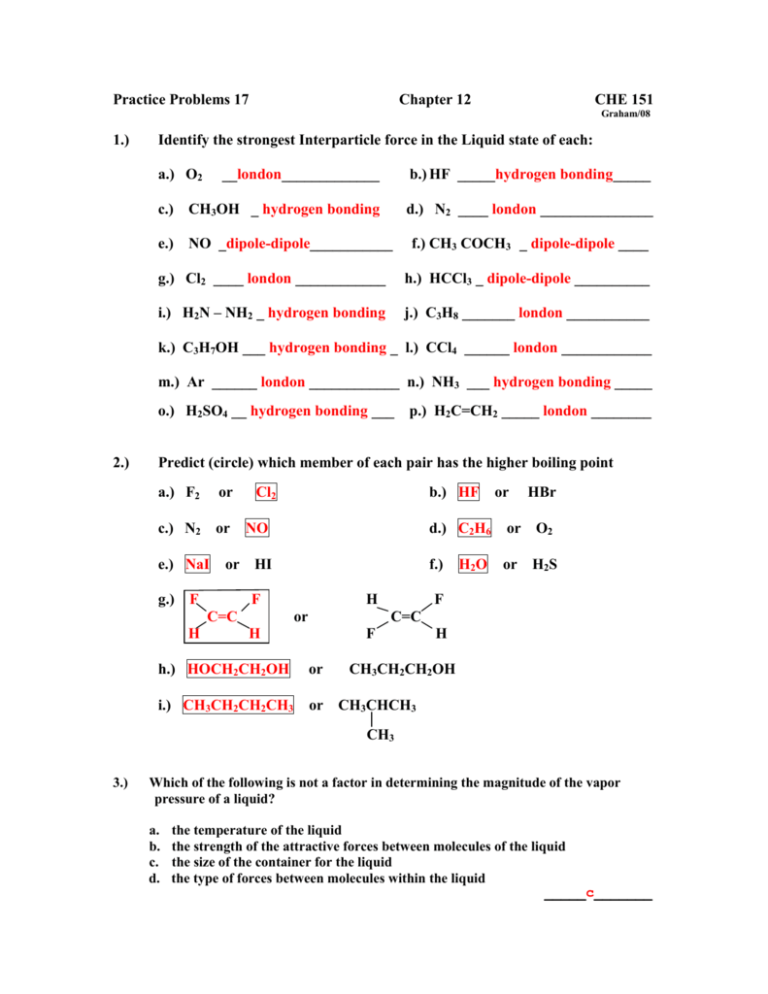
1 H3C-O-CH3 2 H2O 3 CH3CH2OH 4 CH3CH2SH I think the order should be. B 10 M solution of ionic compound potassium chloride KCl.
FeCl 3 has the highest boiling point.
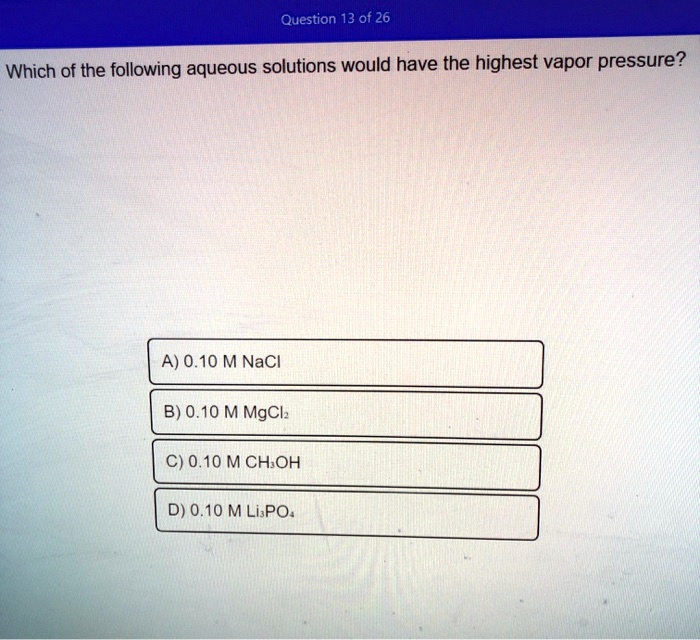
. B 010 M MgCl₂. Water H2O100C methanol CH3OH6496C ethanol CH3CH2OH785C diethyl ether CH3OH2-O-CH2CH3 345C ethylene glycol HO-CH2-CH2-OH198C. The freezing point of a solution made using toluene in benzene is determined to be -130 C.
See answers 2. Which of the following would have the highest vapor pressure. The reason for its high vapour pressure is that the attraction is less between ether molecules than between water and alcohol molecules.
Its vapor pressure at 20C is 5896 kPa. Vapor Pressure and Temperature. If that isnt enough detail Ive covered it in more detail here.
10 M solution of ionic compound sodium chloride NaCl 10 M solution of ionic comopund potassium chloride KCl 10 M solution of molecular compound sucrose C12H22O11 pure water. Liquid A has vapor pressure X and liquid B has vapor pressure Y. The vapor pressure of water at 20C is only 233 kPa far less than that of diethyl ether.
Of particles in solutionions. A CsH2 b MgCl2 c CH-CH3NH2 d PBT e SOCI 4. Which of the following would have the highest vapor pressure.
July 23 2021 July 23 2021 thanh. Therefore B has the higher vapor pressure. Hereof which has the highest vapor pressure.
So in the given compounds we have methanol which has hydrogen bonding which is here. Vapor pressure is dependent upon temperature. A 010 M NaCl.
So here were looking to determine which one has the highest boiling point out of the examples we have. Which one of the following solutions has the highest vapor pressure. Since A is harder to vaporize it has the lower vapor pressure.
C 10 M solution of molecular compound sucrose C12 H22 O11. V P noof particlesions 1 Since glucose is a. D 010 M Li₃PO₄.
Vapor pressure at 25 o C. Which of the following has highest boiling point 01 M urea. The graph shows that propanone has the greatest vapor pressure at any given temperature compared to the other three liquids while ethanoic acid has the lowest vapor pressure at any given temperature compared to the other three liquids.
A 200 g of glucose C6H12O6 in 1000 mL of water. At the normal boiling point of a liquid the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere 760 Torr 101325 kPa or 1469595 psi. So the boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
Which of the following objects has the highest saturation vapor pressure thus making it most difficult for it to grow in size when in the 183184185. Which of the following aqueous solutions would have the highest vapor pressure. Ether has the highest vapour pressure ie lowest boilint point at any temperature.
Ether has the highest vapour pressure ie lowest boilint point at any temperature. Water is a polar liquid whose molecules are attracted to one another by relatively strong hydrogen bonding. Which of the following has the highest vapor pressure.
B 200 g of sucrose C12H22O11 in 1000 mL of water. Vapour Pressure of equimolar solutions is inversely proportional to no. A CsH2 b CH24 c CHIA d C10H22 e C12H26 2.
A 10 M solution of ionic compound sodium chloride NaCl. Which of the following liquids will have the highest vapor pressure. For example at any given temperature methyl chloride has the highest vapor pressure of any of the liquids in the chart.
Which of the following should have the highest surface tension. A CH32N b CH OCH c CH-CH2OH d CHCHF 3. Which of the following should have the lowest vapor pressure.
The reason for its high vapour pressure is that the attraction is less between ether molecules than between water and alcohol molecules. PH3 SiH4 H2Se GeH4 HF. Which one of the liquids would you expect to have the highest vapor pressure at room temperature.
Water bp 100 C benzene bp 80 C chloroform bp 61 C acetone bp 56 C explanation. Which of the following has the highest vapor pressure. Arrange the compounds in the order of increasing boiling point LOWEST first.
The lowest boiling point will have the highest vapor pressure. Presence of the others. See the answer See the answer done loading.
Which of the following should have the lowest boiling point. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid or solid. 1 4 3 2 Arrange the following in order of increasing rate of reactivity.
That is the pressure of the vapor resulting from evaporation of a liquid or solid above a sample of the liquid or solid in a closed container. The freezing point of pure benzene is 549 C. C 010 M CH₃OH.
Because it is ionic and will have highest number of solute particles which will decrease the vapour pressure and increase the boiling point of the solution.
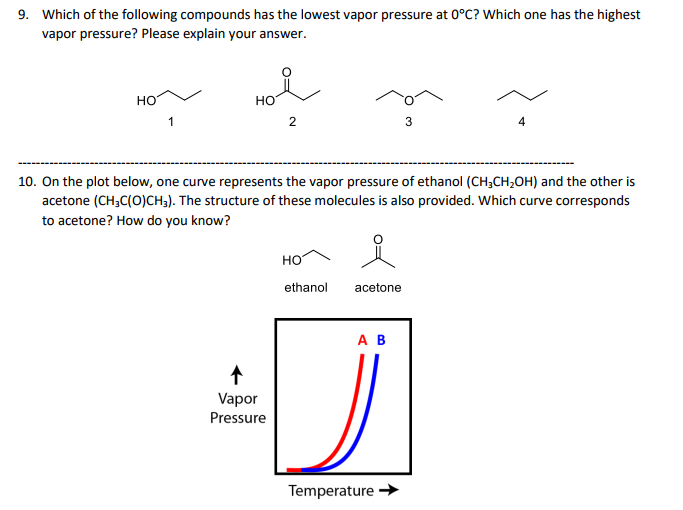
Solved Which Of The Following Compounds Has The Lowest Vapor Chegg Com

Which Molecules Have Higher Or Lower Vapor Pressure Youtube

Solved Question 13 Of 26 Which Of The Following Aqueous Solutions Would Have The Highest Vapor Pressure A 0 10 M Nacl B 0 10 M Mgclz C 0 10 Mchoh D 0 10 M Lispo

Question Video Determining Which Solution Will Have The Lowest Vapor Pressure Nagwa




Belum ada Komentar untuk "Which of the Following Would Have the Highest Vapor Pressure"
Posting Komentar